

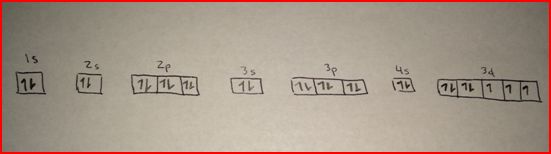
The absence or low presence of cobalt can cause anemia.Ĭobalamin proteins use the corrin ring to hold the cobalt unit. The Electronic configuration of Co: Ar 3d7 4s2. Cobalt is a transition metal and the atomic symbol of Cobalt is Co. It makes up cobalamin, also called vitamin B12. Electronic configuration: It is the arrangement of electrons in the increasing order of energy in the shells of an atom. Biological function of cobaltĬobalt is needed in all animals and in humans as well. In the absence of nuclear war, the risk arises from improper maintenance or handling of radiotherapy units. This is why certain nuclear weapons called dirty bombs are created.
The source used has a radius of 2 cm and produces the presence of penumbral parts that diffuse the radiation that surrounds its direction. it is therefore difficult to be able to encapsulate it to avoid radioactive contamination.Ĭobalt-60 generates two gamma rays that have energies of 1.33 MeV and 1.17 Mev. Isotopes lighter than the stable Co-59 are primarily decayed by electron capture creating iron isotopes, while those heavier than the stable isotope are decayed by beta emission giving rise to nickel isotopes.Ĭobalt-60 is used in radiotherapy instead of radium because of its economic price and also because radium decays into radon and it is a radioactive element in the form of a gas. The atomic mass of isotopes of this element is between 50 amu and 73 amu. Cobalt also has four meta-states, each of which has half-lives of less than 15 minutes. The other radioactive isotopes have half-lives of less than 18 hours and almost all of them less than one second. The most stable are Co-56, Co-57 and Co-60 whose half-lives are 70.86 days, 271.79 days and 5.2714 years respectively. The boiling point of this element is 2927.85 degrees Celsius or 3200 Kelvin and the melting point is 1495.85 degrees Celsius or 1768 Kelvin. This chemical element has a gray tint, is metallic and belongs to the group of transition metals, its atomic number is 27 and it is represented by the symbol Co.
Full electron configuration of cobalt free#
You have signed an examinee agreement, and it will be enforced on this subreddit.ĭo not intentionally advertise paid or free products or services of any sort.The covalent radius of cobalt is 126 pm, the Bohr radius or atomic radius is 152 pm, while the average radius is 135 pm.Ĭobalt is solid in its natural form, specifically a ferromagnetic solid. We have one "stickied" post for each exam and score release day, contain all test day discussion/reactions to that thread only.ĭo not discuss any specific information from your actual MCAT exam. For an example format for submitting pictures of questions from practice material click hereĭo not link to content that infringes on copyright laws (MCAT torrents, third party resources, etc).ĭo not post repeat "GOOD LUCK", "TEST SCORE", or test reaction posts. These are considered spoilers and should be marked as such. Be nice to each other, hating on other users won't help you get extra points on the MCAT, so why do it?ĭo not post any question information from any resource in the title of your post. Rudeness or trolling will not be tolerated. Please message the moderators with your skills/ideas! MCAT RESOURCES & INFO Study Groups Want to help us improve this subreddit or tell us about a new resource we can add to the sidebar? Below you will find our forum rules, resources, and more. We request that you read the sidebar COMPLETELY before you post. r/MCAT is a place for support, discussion, advice, social networking, news, study tips and more. The MCAT (Medical College Admission Test) is offered by the AAMC and is a required exam for admission to medical schools in the USA and Canada. Welcome to the BEST place for MCAT prep and practice materials.


 0 kommentar(er)
0 kommentar(er)
